2019 report advancing health through innovation
December 1 , 2019
In 2019, the FDA approved a wide variety of new drug therapies to help patients in need.
Happy New Year! The beginning of a new year is always a rewarding time for the many dedicated staff at the FDA's Center for Drug Evaluation and Research (CDER) who work on approving new drug therapies. We look back over the past year and see how the work we did came together to help patients in need — one of the main reasons why many of us, including me, have chosen a career in regulatory science and public health.
In 2019, we approved a wide variety of new drugs never before marketed in the United States, known as "novel" drugs, along with a range of new approvals containing active ingredients already on the market put to new and innovative uses. Many will have a positive and even life-saving impact, on countless patients' lives.
Many More Rare Disease Approvals in Recent Years
New drug therapies for patients suffering from rare diseases are often among the most important approvals. Patients with rare diseases frequently have few or no drugs available to treat their condition — and for them, approvals of so-called “orphan” drugs can mean new hope for an enhanced quality of life, and in some cases, survival.
Among others, in 2019 CDER approved new therapies for patients suffering from a wide array of rare and debilitating conditions including cystic fibrosis, systemic sclerosis-associated interstitial lung disease, erythropoietic protoporphyria, neuromyelitis optica spectrum disorder, and Duchenne muscular dystrophy.
Many orphan drug approvals can help patients with various forms of cancer, tumors, and blood disorders. In 2019, among others, CDER approved new drug therapies for patients with tenosynovial giant cell tumor, sickle cell disease, diffuse large B-cell lymphoma, mantle cell lymphoma, a type of myelofibrosis, acquired thrombotic thrombocytopenic purpura, beta-thalassemia, and acute hepatic porphyria.
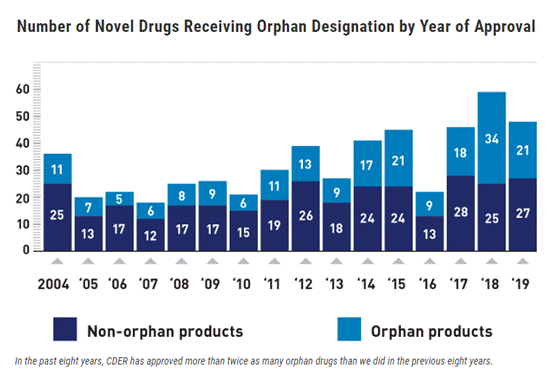
In 2019, 21 of CDER’s 48 novel drug approvals, or 44%, were orphan drugs. Like other recent years, 2019 shows a significant increase in CDER’s orphan drug approvals compared to earlier years. The graph below shows that CDER approved more than twice as many novel orphan drugs from 2012-2019 than in the previous 8-year period from 2004-2011 — 142 vs. 63, a 125% increase.
Neurological, Infectious Disease, Endocrinology, Autoimmune Disorders — and More
In addition to CDER’s approval of new therapies to treat patients with rare diseases, we approved many other important new therapies in 2019. These include:
Neurological advances in therapies to treat patients with multiple sclerosis, depression, episodic cluster headaches, “off episodes” of Parkinson's disease, and migraine.
Infectious Disease innovations such as two new antibiotics effective against certain Gram-negative infections (ofte
n resistant to treatment), a new antibiotic for the treatment of patients with community-acquired bacterial pneumonia, and advances in the treatment and prevention of HIV.
Endocrinology advances to treat patients with type 2 diabetes, including a new nasal powder to treat patients with severe hypoglycemia and the first medication in the drug class, “glucagon-like peptide receptor protein,” approved for use in the U.S. that does not need to be injected.
Innovative new therapies for certain patients with autoimmune conditions that include non-radiographic axial spondyloarthritis, which causes inflammation in the spine and other symptoms, and Lambert-Eaton myasthenic syndrome, in which the immune system attacks the body's own tissues.
Women’s and men’s health innovations that include a new treatment for osteoporosis in certain postmenopausal women at high risk of breaking a bone, a new drug to treat low sexual desire in premenopausal women, the first FDA-approved drug specifically for the treatment of adult women with postpartum depression, and a new therapy for an oral testosterone capsule to treat men with low testosterone levels due to specific medical conditions. (Until this approval, testosterone replacement therapies have most commonly been applied to the skin or injected).
Of note, we also approved ten new biosimilars, which can further help to create competition, increase patient access, and potentially reduce the cost of important biological drug therapies.
Efficiencies in Getting New Therapies to Patients as Quickly as Possible
It is also gratifying to take a look back at 2019 and appreciate the efficiencies in our review of these newly approved therapies. The decisions we made on these approvals were generally completed by or before their goal dates as defined by Congressionally-mandated user fee programs — a high-priority commitment for the FDA. Most were approved in the United States before any other country in the world.
Meeting User Fee Goals: In 2019, CDER met its Prescription Drug User Fee Act (PDUFA) goal dates for 100% of the novel drugs approved (48 of 48).
First Cycle Approvals: In 2019, CDER approved 43 of our 48 novel approvals (90%) on the first cycle. A first cycle is the time from when CDER accepts an application for a new drug until we make the first decision to approve or not approve (issue a complete response letter) the application. From 2011 through 2018, CDER approved 309 novel drugs, of which 261 (84%) were approved on the first cycle. Our consistently high first cycle approval rate for new drugs reflects CDER’s commitment to working closely with applicants as they design their studies and build their application data.
Approvals Before Other Countries: Although regulatory processes differ widely between the FDA and those of regulatory agencies in other countries, 33 of the 48 novel drugs approved in 2019 (69%) were approved in the United States before receiving approval in any other country.
Expedited Programs for Serious Conditions: CDER houses four programs intended to facilitate and expedite development and review of new drugs to significantly advance treatment or address unmet medical need for a serious or life-threatening condition: fast track, breakthrough designation, priority review designation, and accelerated approval. In 2019, 60% of CDER’s novel drug approvals (29 of 48) used one or more of these expedited programs, bringing new therapies to patients months, or even years, sooner than expected.
Maintaining CDER’s High Approval Standards
Over the years, as the number of orphan drugs, “targeted” therapies (sometimes called “personalized medicines”), cancer treatments, and other medical advances being developed and reviewed has increased, so too has the need to tailor drug development programs and review to these particular therapies, as well as the use of FDA’s expedited drug development and review programs to help potentially bring a wide range of new drugs to patients in need. In the past four years, from 2016 through 2019, of the 175 novel drugs CDER approved, 116 or 66%, used at least one expedited development program. By comparison, in the four years from 2012 through 2015, of the 152 novel drugs CDER approved, 89 or 59%, used at least one expedited development program.
The graph above illustrates the growth in development and approval of new orphan drugs. This includes a growing number of CDER’s approvals each year that are targeted therapies, meaning drugs that are effective only for a population of patients with certain genetic characteristics — a trend that is certain to continue as the field of genetics continues to expand. While many targeted therapies are developed to treat patients with rare diseases, many others are not orphan products. It is important to recognize that drugs developed for specific populations of individuals (i.e., orphan drugs and targeted therapies) are often candidates for expedited programs.
Likewise, the portfolio of cancer drugs, many for people lacking other options, has also rapidly grown — to currently comprise more than a third of CDER’s novel drug approvals.
As the use of tools intended to expedite the development and review of needed medications increases, our high standards for safety and efficacy have remained unchanged.
More details about CDER’s new drug therapy approvals — including many specific examples of notable new approvals for the year — are available in our annual New Drug Therapy Approvals report.
I am proud to lead a team of dedicated professionals working to bring new therapies to patients as quickly as possible, while at the same time ensuring that approval for each and every one of these advances is based on our same high standards for science, safety and efficacy. As 2020 begins, we look forward to another strong year of safely advancing health care for the American public.
Happy New Year! The beginning of a new year is always a rewarding time for the many dedicated staff at the FDA's Center for Drug Evaluation and Research (CDER) who work on approving new drug therapies. We look back over the past year and see how the work we did came together to help patients in need — one of the main reasons why many of us, including me, have chosen a career in regulatory science and public health.
In 2019, we approved a wide variety of new drugs never before marketed in the United States, known as "novel" drugs, along with a range of new approvals containing active ingredients already on the market put to new and innovative uses. Many will have a positive and even life-saving impact, on countless patients' lives.
Many More Rare Disease Approvals in Recent Years
New drug therapies for patients suffering from rare diseases are often among the most important approvals. Patients with rare diseases frequently have few or no drugs available to treat their condition — and for them, approvals of so-called “orphan” drugs can mean new hope for an enhanced quality of life, and in some cases, survival.
Among others, in 2019 CDER approved new therapies for patients suffering from a wide array of rare and debilitating conditions including cystic fibrosis, systemic sclerosis-associated interstitial lung disease, erythropoietic protoporphyria, neuromyelitis optica spectrum disorder, and Duchenne muscular dystrophy.
Many orphan drug approvals can help patients with various forms of cancer, tumors, and blood disorders. In 2019, among others, CDER approved new drug therapies for patients with tenosynovial giant cell tumor, sickle cell disease, diffuse large B-cell lymphoma, mantle cell lymphoma, a type of myelofibrosis, acquired thrombotic thrombocytopenic purpura, beta-thalassemia, and acute hepatic porphyria.
In 2019, 21 of CDER’s 48 novel drug approvals, or 44%, were orphan drugs. Like other recent years, 2019 shows a significant increase in CDER’s orphan drug approvals compared to earlier years. The graph below shows that CDER approved more than twice as many novel orphan drugs from 2012-2019 than in the previous 8-year period from 2004-2011 — 142 vs. 63, a 125% increase.

Neurological, Infectious Disease, Endocrinology, Autoimmune Disorders — and More
In addition to CDER’s approval of new therapies to treat patients with rare diseases, we approved many other important new therapies in 2019. These include:
Neurological advances in therapies to treat patients with multiple sclerosis, depression, episodic cluster headaches, “off episodes” of Parkinson's disease, and migraine.
Infectious Disease innovations such as two new antibiotics effective against certain Gram-negative infections (ofte
n resistant to treatment), a new antibiotic for the treatment of patients with community-acquired bacterial pneumonia, and advances in the treatment and prevention of HIV.
Endocrinology advances to treat patients with type 2 diabetes, including a new nasal powder to treat patients with severe hypoglycemia and the first medication in the drug class, “glucagon-like peptide receptor protein,” approved for use in the U.S. that does not need to be injected.
Innovative new therapies for certain patients with autoimmune conditions that include non-radiographic axial spondyloarthritis, which causes inflammation in the spine and other symptoms, and Lambert-Eaton myasthenic syndrome, in which the immune system attacks the body's own tissues.
Women’s and men’s health innovations that include a new treatment for osteoporosis in certain postmenopausal women at high risk of breaking a bone, a new drug to treat low sexual desire in premenopausal women, the first FDA-approved drug specifically for the treatment of adult women with postpartum depression, and a new therapy for an oral testosterone capsule to treat men with low testosterone levels due to specific medical conditions. (Until this approval, testosterone replacement therapies have most commonly been applied to the skin or injected).
Of note, we also approved ten new biosimilars, which can further help to create competition, increase patient access, and potentially reduce the cost of important biological drug therapies.
Efficiencies in Getting New Therapies to Patients as Quickly as Possible
It is also gratifying to take a look back at 2019 and appreciate the efficiencies in our review of these newly approved therapies. The decisions we made on these approvals were generally completed by or before their goal dates as defined by Congressionally-mandated user fee programs — a high-priority commitment for the FDA. Most were approved in the United States before any other country in the world.
Meeting User Fee Goals: In 2019, CDER met its Prescription Drug User Fee Act (PDUFA) goal dates for 100% of the novel drugs approved (48 of 48).
First Cycle Approvals: In 2019, CDER approved 43 of our 48 novel approvals (90%) on the first cycle. A first cycle is the time from when CDER accepts an application for a new drug until we make the first decision to approve or not approve (issue a complete response letter) the application. From 2011 through 2018, CDER approved 309 novel drugs, of which 261 (84%) were approved on the first cycle. Our consistently high first cycle approval rate for new drugs reflects CDER’s commitment to working closely with applicants as they design their studies and build their application data.
Approvals Before Other Countries: Although regulatory processes differ widely between the FDA and those of regulatory agencies in other countries, 33 of the 48 novel drugs approved in 2019 (69%) were approved in the United States before receiving approval in any other country.
Expedited Programs for Serious Conditions: CDER houses four programs intended to facilitate and expedite development and review of new drugs to significantly advance treatment or address unmet medical need for a serious or life-threatening condition: fast track, breakthrough designation, priority review designation, and accelerated approval. In 2019, 60% of CDER’s novel drug approvals (29 of 48) used one or more of these expedited programs, bringing new therapies to patients months, or even years, sooner than expected.
Maintaining CDER’s High Approval Standards
Over the years, as the number of orphan drugs, “targeted” therapies (sometimes called “personalized medicines”), cancer treatments, and other medical advances being developed and reviewed has increased, so too has the need to tailor drug development programs and review to these particular therapies, as well as the use of FDA’s expedited drug development and review programs to help potentially bring a wide range of new drugs to patients in need. In the past four years, from 2016 through 2019, of the 175 novel drugs CDER approved, 116 or 66%, used at least one expedited development program. By comparison, in the four years from 2012 through 2015, of the 152 novel drugs CDER approved, 89 or 59%, used at least one expedited development program.
The graph above illustrates the growth in development and approval of new orphan drugs. This includes a growing number of CDER’s approvals each year that are targeted therapies, meaning drugs that are effective only for a population of patients with certain genetic characteristics — a trend that is certain to continue as the field of genetics continues to expand. While many targeted therapies are developed to treat patients with rare diseases, many others are not orphan products. It is important to recognize that drugs developed for specific populations of individuals (i.e., orphan drugs and targeted therapies) are often candidates for expedited programs.
Likewise, the portfolio of cancer drugs, many for people lacking other options, has also rapidly grown — to currently comprise more than a third of CDER’s novel drug approvals.
As the use of tools intended to expedite the development and review of needed medications increases, our high standards for safety and efficacy have remained unchanged.
More details about CDER’s new drug therapy approvals — including many specific examples of notable new approvals for the year — are available in our annual New Drug Therapy Approvals report.
I am proud to lead a team of dedicated professionals working to bring new therapies to patients as quickly as possible, while at the same time ensuring that approval for each and every one of these advances is based on our same high standards for science, safety and efficacy. As 2020 begins, we look forward to another strong year of safely advancing health care for the American public.
