The top 10 drugs losing U.S. exclusivity in 2020
March 1 , 2020
Fiercepharma, Mar. 2020
Each year, drugmakers face costly losses of exclusivity for big-selling old medicines, forcing them to continually develop and launch new drugs as they strive for growth. While some major brands like Lyrica and Herceptin lost exclusivity last year—with more expected for the coming years—2020 will feature the introduction of important new generics, as well.
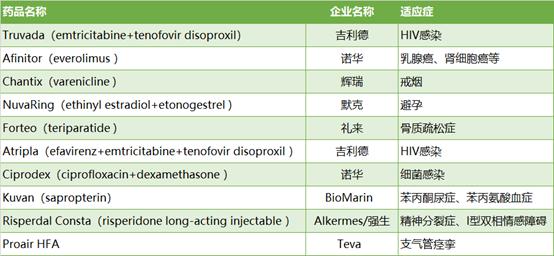
Truvada, Gilead’s HIV drug that’s been in the news a lot lately, is set to top the list. The drug pulled in $2.6 billion in the U.S. last year, and under a patent deal with Teva Pharmaceuticals, it’ll face new generics in September. Meanwhile, Gilead is locked in a dispute with the U.S. government on HIV prevention patents on the medicine.
Aside from Truvada, 2020’s top U.S. losses of exclusivity include medications from Novartis, Bristol-Myers Squibb, Merck, Eli Lilly and others. They’re drugs already facing new generics under year-end 2019 launches, plus those expected to lose exclusivity later in the year. The list totals some $8 billion in 2019 U.S. sales.
Meanwhile, Allergan’s Restasis is one drug that just keeps dodging U.S. competition—even after its patents expired—thanks to regulatory struggles for generics companies. Teva has had “positive discussions” lately, an executive said in February, but the timing for its launch remains unknown.
Down the line, many in the industry are tracking the 2023 downfall of AbbVie’s Humira protection. The drug is so important to AbbVie that the drugmaker inked a massive buyout in Allergan to help prepare for that patent cliff. Roche’s Lucentis and Novartis’ Gilenya might also face generics or biosims soon, but those copycats don’t seem likely in 2020 as of now.
Biogen’s Tecfidera and Amgen’s Enbrel have also dodged immediate competition, thanks to a win at the U.S. Patent & Trademark Office and in federal court, respectively. And Takeda’s Dexilant is set to face competition sometime in the coming years, but Endo says the terms of its patent settlement with the Japanese drugmaker are confidential.
With patent lawsuits, settlements, FDA setbacks and other constantly changing factors, it’s impossible to predict generic entry in many cases. This report is intended to be a point-in-time look at likely losses of exclusivity based on public filings, company statements and FDA records.
If you would like to read back to previous versions of the report, you can find them here for 2019 and here for 2018. And as always, let us know your thoughts. — Eric Sagonowsky Email | Twitter

Each year, drugmakers face costly losses of exclusivity for big-selling old medicines, forcing them to continually develop and launch new drugs as they strive for growth. While some major brands like Lyrica and Herceptin lost exclusivity last year—with more expected for the coming years—2020 will feature the introduction of important new generics, as well.
Truvada, Gilead’s HIV drug that’s been in the news a lot lately, is set to top the list. The drug pulled in $2.6 billion in the U.S. last year, and under a patent deal with Teva Pharmaceuticals, it’ll face new generics in September. Meanwhile, Gilead is locked in a dispute with the U.S. government on HIV prevention patents on the medicine.
Aside from Truvada, 2020’s top U.S. losses of exclusivity include medications from Novartis, Bristol-Myers Squibb, Merck, Eli Lilly and others. They’re drugs already facing new generics under year-end 2019 launches, plus those expected to lose exclusivity later in the year. The list totals some $8 billion in 2019 U.S. sales.
Meanwhile, Allergan’s Restasis is one drug that just keeps dodging U.S. competition—even after its patents expired—thanks to regulatory struggles for generics companies. Teva has had “positive discussions” lately, an executive said in February, but the timing for its launch remains unknown.
Down the line, many in the industry are tracking the 2023 downfall of AbbVie’s Humira protection. The drug is so important to AbbVie that the drugmaker inked a massive buyout in Allergan to help prepare for that patent cliff. Roche’s Lucentis and Novartis’ Gilenya might also face generics or biosims soon, but those copycats don’t seem likely in 2020 as of now.
Biogen’s Tecfidera and Amgen’s Enbrel have also dodged immediate competition, thanks to a win at the U.S. Patent & Trademark Office and in federal court, respectively. And Takeda’s Dexilant is set to face competition sometime in the coming years, but Endo says the terms of its patent settlement with the Japanese drugmaker are confidential.
With patent lawsuits, settlements, FDA setbacks and other constantly changing factors, it’s impossible to predict generic entry in many cases. This report is intended to be a point-in-time look at likely losses of exclusivity based on public filings, company statements and FDA records.
If you would like to read back to previous versions of the report, you can find them here for 2019 and here for 2018. And as always, let us know your thoughts. — Eric Sagonowsky Email | Twitter


